Your new post is loading...
 Your new post is loading...
Saliva may be a reliable and cost-efficient way to diagnose COVID-19, according to a study published recently in the Journal of Evidence-Based Dental Practice. Saliva-based tests were found to be roughly as reliable as nasopharynx or throat swabs.
Human cytomegalovirus (HCMV) infections are common following renal transplantation and may have long-lasting effects. HCMV can be measured directly by viral DNA or indirectly via host immune responses.
SalivaDirect is a simple, affordable, and less invasive testing method that has been found to yield similar outcomes to the traditional nasal swab test. ...
The FDA authorized its first COVID-19 diagnostic test that allows a person to collect a simple saliva sample themselves, without leaving their home, similar to a personal DNA test. The green light expands upon a previous authorization for a test developed by Rutgers University, designed to use a person’s spit instead of a nasal swab. The agency also granted its blessing to LabCorp’s at-home swab test in late April. “Authorizing additional diagnostic tests with the option of at-home sample collection will continue to increase patient access to testing for COVID-19,” FDA Commissioner Stephen Hahn said in a statement. “This provides an additional option for the easy, safe and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site.” “The FDA has authorized more than 80 COVID-19 tests and adding more options for at-home sample collection is an important advancement in diagnostic testing during this public health emergency,” Hahn added. Rutgers’ prescription-only molecular test currently remains as the only diagnostic authorized by the FDA to process saliva samples. After using a collection kit made by Spectrum DNA, the sealed package is then mailed to the New Jersey university’s clinical genomics lab for analysis. Previously, the university said that quickly administered, saliva-based tests could find equally important use in the field—not just in the home—by sparing healthcare workers exposure from collection methods that require them to manually swab a person’s deep nasal cavity....
Via Juan Lama
The use of saliva samples in clinical studies has increased. However, the diagnostic value of whole saliva is compromised in the presence of blood contamination...
|
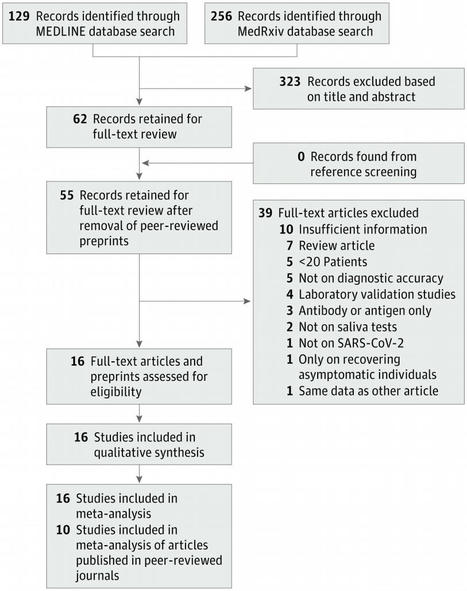
This systematic review and meta-analysis assess the diagnostic accuracy of saliva nucleic acid amplification tests for diagnosis of coronavirus disease 2019.
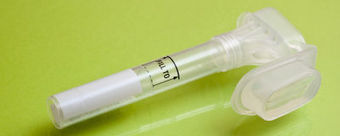
Science's COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation A woman spits into a tube so that her saliva can be tested for the presence of the novel coronavirus. PHOTO: UNIVERSITY OF ILLINOIS, URBANA-CHAMPAIGN First, a technician pushes a pencil-length swab to the very back of your nasal passages. Then you pay $100 or more, and wait days for an answer. But faster, cheaper, more pleasant ways to test for the novel coronavirus are coming online. This month, the U.S. Food and Drug Administration granted emergency use authorization for two tests that sample saliva instead of nasal fluid, and more innovations are likely after FDA relaxed rules to allow new tests to be adopted more quickly. One candidate was announced last week: an experimental test, potentially faster and cheaper, that analyzes saliva in a new way. “There is real promise here,” says Anne Wyllie, a microbiologist at Yale University who helped develop one of the new tests authorized this month. Takanori Teshima, chief of laboratory medicine at Hokkaido University, who also reported successful results testing saliva, agrees. Saliva tests “will have a big impact worldwide.” When SARS-CoV-2, the respiratory virus that causes COVID-19, emerged in December 2019, researchers scrambled to develop tests to detect the virus. Initially, they turned to a long-trusted technique for diagnosing respiratory infections: looking for viral genetic material in mucosal fluid, thought to be the best hunting ground for a respiratory virus, collected from deep in a patient's nasal passages. That's where the 15-centimeter swab comes in. The swab goes into a plastic tube with a chemical mixture that stabilizes the virus during transport to a diagnostics lab. There, technicians extract its genetic material and load it into a machine to carry out the polymerase chain reaction (PCR), which amplifies snippets of genetic material unique to the virus. The procedure accurately identifies infections about 95% of the time. But the test is uncomfortable and, because collecting the swab requires close contact with patients, it puts medical personnel at risk of contracting the virus. “Nobody wants to do that job,” Teshima says. Testing saliva for SARS-CoV-2 was no sure thing. Studies with other respiratory diseases showed saliva tests identified only about 90% of people for whom swab tests indicated an infection. But the appeal of an easier and safer test for the new coronavirus led researchers to try. People being tested simply drool into a bar-coded plastic tube, seal it, and drop it in a pouch that's shipped to a lab for PCR analysis. Because the procedure directly tests the fluid responsible for transmitting the virus between people, it may give a better indication of who is most contagious, says Paul Hergenrother, a chemist at the University of Illinois, Urbana-Champaign (UIUC), who led his university's saliva test development. As early as 12 February, researchers in Hong Kong and China reported in Clinical Infectious Diseases that they could identify SARS-CoV-2 from saliva in 11 of 12 patients whose swabs showed virus. Since then, groups in the United States, Singapore, and Japan have confirmed and further simplified the procedures, cutting out costly steps such as adding specialized reagents to stabilize the virus and extract the genetic material. In May, Wyllie and Yale colleagues teamed up with the National Basketball Association, which provided $500,000 to develop Yale's saliva diagnostic; the test is now used for frequently testing players. On 4 August, the Yale team posted a preprint on medRxiv that said its saliva test agreed with swab results 94% of the time, at a cost of as little as $1.29 per sample, roughly 1/100 as much as commercial swab-based tests. On 15 August, FDA granted emergency approval for the test, called SalivaDirect, so that other FDA-approved labs can use the protocol. Last week, the agency extended approval to the UIUC test given its similarity to the Yale test. UIUC is now using its saliva test with all 60,000 students, faculty, and staff twice a week, in order to isolate infected individuals as quickly as possible. “Testing saliva makes sense scientifically, and it makes sense logistically,” Hergenrother says. A new saliva test for RNA viruses, such as Zika and SARS-CoV-2, was reported last week in Science Advances by researchers at the University at Albany. It could be even faster and cheaper because it does not need expensive lab equipment such as PCR machines. Rather than amplifying RNA to identify the virus, the approach uses snippets of DNA that bind to short, unique sections of RNA and change them from linear strands to loops. That alters how the RNA behaves in a common lab procedure known as gel electrophoresis, making it easy to detect. “This is innovative,” Wyllie says. A relaxation of FDA rules announced last week could lead to still more variants. The new rules allow approved clinical labs to use tests they have developed without any additional approval step. In a tweet, Michael Mina, an epidemiologist at Harvard University's T.H. Chan School of Public Health, called FDA's decision “Huge news!!” because it would encourage labs to develop novel tests. It may also help speed development of rapid tests that look for viral proteins rather than genetic material—an efficient way to screen large numbers of asymptomatic people. “We don't need one test to be the end all and be all,” Wyllie says. “We just want options.”
medRxiv - The Preprint Server for Health Sciences
The search for SARS-CoV-2 RNA in 60 saliva samples yielded the same results as conventional nasal swab tests taken from the same patients. There are now more options for COVID-19 testing as the US Food and Drug Administration gave emergency use authorization on April 13 for a saliva-based test, providing an alternative to the swab testing currently performed. “You want to be in all types of situations with all types of options so that we can have as much testing as possible in whatever form is suitable,” Amesh Adalja, who works on infectious disease and pandemic preparedness at Johns Hopkins University and is not involved with the development of the test, tells the Associated Press. Currently, testing for COVID-19 involves a healthcare professional inserting a swab into each nostril, one at a time, to the nasopharynx at the back of the nasal cavity, gently scraping the tissue to collect material, and sending off for analysis, according to UC Davis Health. This method is cumbersome as it needs to be done by a qualified worker wearing fresh gloves and other personal protective equipment (PPE), which are in short supply. Additionally, many areas are experiencing a lack of tests available or a large backlog of samples to process. The collection of a saliva sample requires spitting into a tube, resulting in a much less invasive procedure without tying up large amounts of PPE. Per the Food and Drug Administration’s (FDA) instructions, the testing would still occur in a healthcare setting under the supervision of a qualified professional.... Performance Letter by FDA: https://www.fda.gov/media/136875/download
Via Juan Lama
Treating Diabetes - a major scourge of humanity bothering millions of people - requires a constant monitoring of the human blood for glucose concentrations.
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...